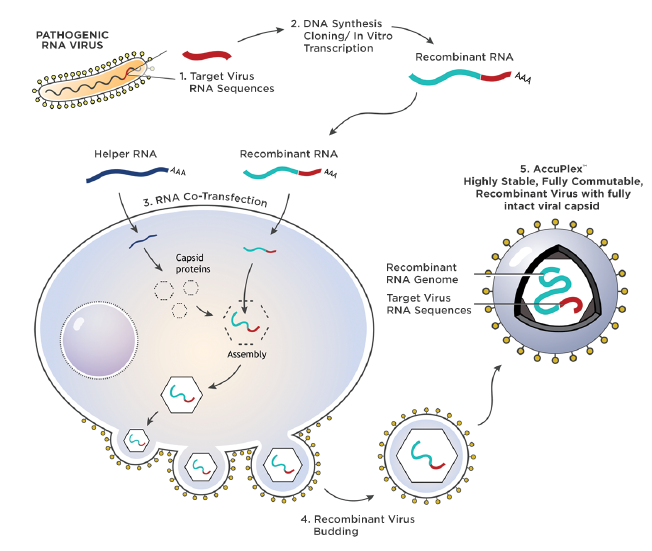
AccuPlex SARS-CoV-2, Flu A/B, and RSV Molecular Controls
Last updated: 30th November, 2021
Products are for professional/laboratory use only. AccuPlex SARS-CoV-2, Flu A/B, and RSV Molecular Controls Kit here are some of the highlights for these new products:
Improved Features:
- CE IVD regulatory clearance (for Molecular Control Kit)
- Developed under full design control and more rigorous performance standards
- Expanded RSV genome coverage (for both products):
– ~2800bp addition – 75% to 90% coverage
– Expanded compatibilit
Kit Details for AccuPlex SARS-CoV-2, Flu A/B, and RSV Molecular Controls Kit:
SeraCare Material Number: BB05050260
Kit Contents:
- Positive Reference Material
– Format: AccuPlex-packaged SARS-CoV-2, Flu and RSV sequences
– Pack size: 5 Vials Positive; 1.5 mL Fill Volume
– Concentration: 5000 copies/mL
- Negative Reference Material
– Format: AccuPlex-packaged RNase P
– Pack size: 5 Vials Negative; 1.5 mL Fill Volume
– Concentration: 5000 copies/mL
Storage: 2 – 8◦C
Shelf Life: 2 Years from Date of Manufacture
Kit Details for AccuPlex SARS-CoV-2, Flu A/B and RSV Verification Panel Version 2:
SeraCare Material Number: BB05050278
Kit Contents:
- Positive Reference Material
– Format: AccuPlex-packaged SARS-CoV-2, Flu and RSV sequences
– Pack size: 3 Vials Positive; 3 mL Fill Volume
– Concentration: 3 Concentration Levels – 1000 copies/mL; 10,000 copies/mL; 100,000 copies/mL
- Negative Reference Material
– Format: AccuPlex-packaged RNase P
– Pack size: 1 Vial; 3mL Fill Volume
– Concentration: 5000 copies/mL
Storage: 2 – 8◦C
Shelf Life: 2 Years from Date of Manufacture