Asuragen revolutionise testing of the SMN1 and SMN2 genes
Products are for professional/laboratory use only.
The AmplideX® PCR/CE SMN1/2 Plus Kit* is an in vitro nucleic acid amplification kit intended to aid in the screening of carriers for and diagnosis of spinal muscular atrophy (SMA).
Asuragen’s AmplideX PCR/CE SMN1/2 Plus Kit*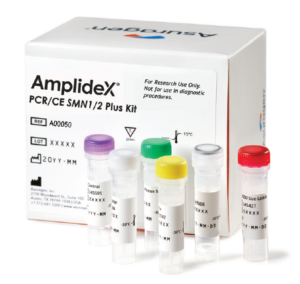
The kit quantifies the number of copies of exon 7 of both SMN1 and SMN2 reported as 0, 1, 2, 3, or ≥ 4 genomic copies. The kit is designed for PCR with extracted genomic DNA from human whole blood performed on standard laboratory-validated thermal cyclers, followed by resolution on a general laboratory-validated genetic analyzer or capillary electrophoresis (CE) platform.
Additionally, the kit identifies chimeric genes with both SMN1 and SMN2 sequences, and detects variants SMN1 c.*3+80T>G and SMN1 c.*211_*212del, which are associated with SMN1 gene duplication and “silent carrier” status, as well as variant SMN2 c.859G>C, which is associated with a milder disease phenotype.
Why choose Amplidex?
Reduced Complexity
Ease of data analysis and reporting
- One kit to identify SMA patients, carriers (including detection of variants associated with silent carriers), and refine disease prognosis – all from a single PCR reaction
- Similar workflow to AmplideX PCR/CE FMR1 kit eases implementation and training
- Assay-specific software automates results reporting and streamlines data analysis
Optimized Workflow
Reduces valuable operator hands-on-time and overall turnaround time
- Diagnostic and screening results are reported in less than four hours with only 60 minutes of hands-on-time
- Scalable workflow supports high sample throughput testing
- Optimized for use on widely installed CE equipment
Quality Performance
Comprehensive analysis of SMN1 and SMN2 genes for the diagnosis and screening of SMA
- High resolution of SMN1/2 copy number across a broad range improves accuracy in identifying SMA patients and carriers
- Excellent concordance of copy number and variant results compared to multiple orthogonal test methods
Analytical Performance
- Automated and streamlined reporting of SMN1 and SMN2 copy number and variant detection via AmplideX Reporter Software
- Provide critical test results from a single reaction — and all in less than four hours
- Excellent concordance of SMN1 and SMN2 copy number results to sites’ in-house methods for over 400 replicates
- High assay specificity permits detection of SMN1–SMN2 hybrid peaks, including those resulting from gene conversion events
- 100% agreement observed between Sanger sequencing and AmplideX SMA Plus Kit for detection of SMN1 gene duplication and SMN2 disease modifier variants
Related products
*Research use only