Biocartis – Clinical utility of Idylla EGFR
THESE PRODUCTS ARE NOT AVAILABLE FOR PURCHASE BY THE GENERAL PUBLIC.
Within 150 minutes and with less than 2 minutes hands-on time, the fully automated Idylla™ EGFR Mutation Test covers 51 mutations in exons 18–21 in a single cartridge using only 1 FFPE tissue section from metastatic NSCLC showing a high concordance of >95% compared with reference methods.
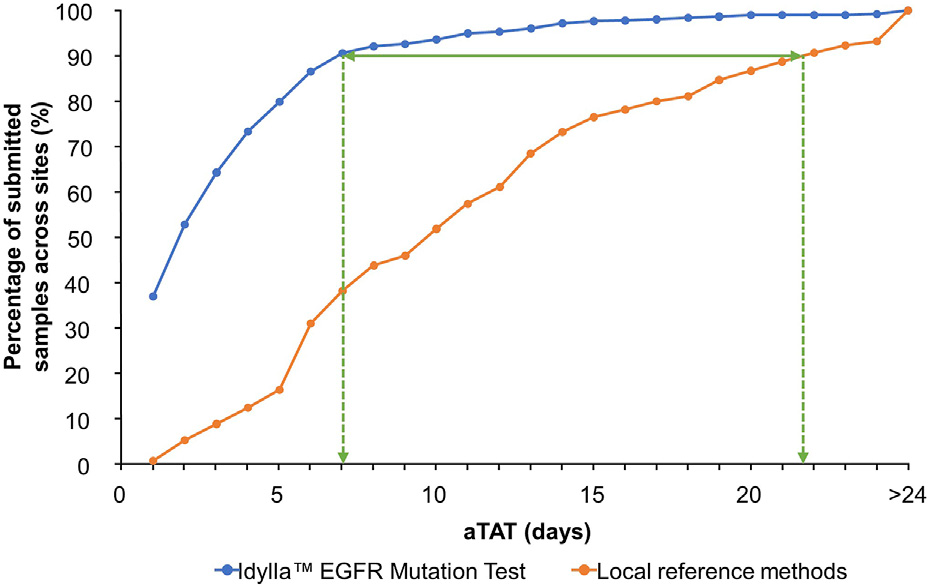
Large FACILITATE Study showing Idylla EGFR mutation results were obtained 2 weeks faster than reference methods.
Behnke et al. – FACILITATE: A real-world multicenter prospective investigating the utility of fully automated real-time PCR assay versus local reference methods for detecting EGFR variants in NSCLC. Pathology & Oncology Research. Jan 2023.
With the Idylla EGFR Mutation Test, 90% of a total of 900 samples with recorded analytical turnaround time data were tested within 7 days. Whereas with reference methods, samples were only tested within approximately 22 days.
Study demonstrating the accuracy and potential clinical utility of the Idylla EGFR Mutation Test as a molecular screening platform.1
Suda et al. – Performance of Ultra-Rapid Idylla EGFR Mutation Test in Non-Small-Cell Lung Cancer and Its Potential at Clinical Molecular Screening.
Cancers (Basel). May 2023.
In this study, the accuracy and potential clinical utility of the Idylla EGFR Mutation Test as a molecular screening platform in terms of turnaround time and molecular testing cost has been demonstrated, both in early stage and advanced NSCLC.
MAIN CONCLUSIONS
- Retrospective observational study in which surgically resected NSCLC specimens obtained at two institutions (N = 170) were examined. The Idylla EGFR Mutation Test and the Cobas EGFR Mutation Test v2 were performed independently and the results were compared. For discordant cases, the Ion AmpliSeq Colon and Lung Cancer Research Panel V2 was used.
- After the exclusion of five inadequate/invalid samples, 165 cases were evaluated. EGFR mutation analysis revealed 52 were positive and 107 were negative for EGFR mutation in both assays (overall concordance rate: 96.4%). Analyses of the six discordant cases revealed that the Idylla EGFR Mutation Test was correct in four cases. Cobas EGFR Mutation Test v2 was correct in two cases and demonstrated a false positive result.
ο All stages of NSCLC were included with a majority in early stage (IA:100; IB: 20, II-III:37 and IV:9) in which low failure rate was demonstrated (1 invalid result due to too low quality, also invalid with Cobas).
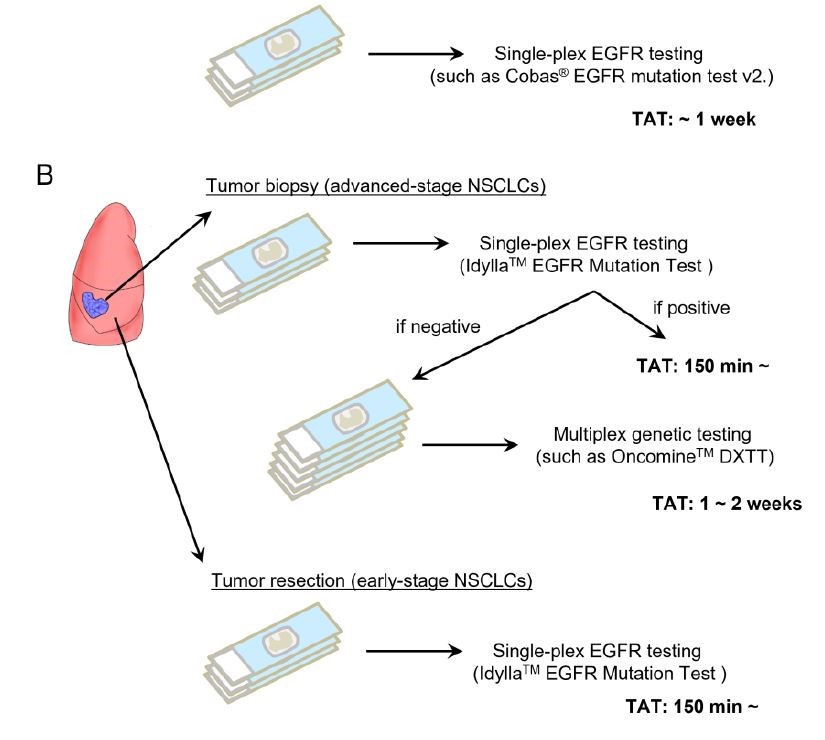
ο The authors suggest a new testing algorithm with Idylla first, for both early stage (single-plex EGFR testing needed) and advanced setting (Idylla followed by NGS when wild-type results).
ο In a cost-trial calculation, the use of Idylla EGFR Mutation Test followed by a multi-gene panel test will reduce molecular screening expenses if applied to a cohort with EGFR mutation frequency >17.9%.
- The authors demonstrated the accuracy and potential clinical utility of the Idylla EGFR Mutation Test as a molecular screening platform in terms of turnaround time and molecular testing cost if applied to a cohort with a high EGFR mutation incidence.
Figure 1. Incorporation of the Idylla EGFR mutation test before multiplex genetic testing in a cohort with high EGFR mutation frequency. (B) Potential strategies to incorporate the Idylla EGFR mutation test.
1 https://www.biocartis.com/en/suda-et-al-global