Invivoscribe NPM1 MRD Assay
Just launched! NPM1 MRD Assay* by Invivoscribe.
The NPM1 MRD test is an NGS-based, targeted, deep-sequencing assay that can detect NPM1 mutations previously identified in a primary sample.
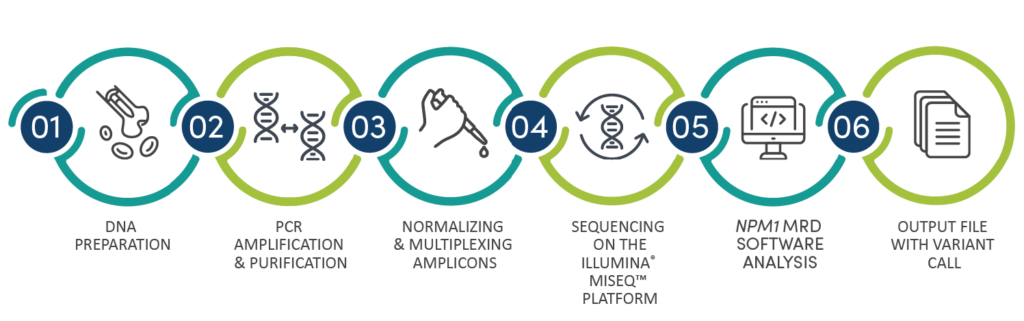
To identify and track previously detected NPM1 mutations in post-treatment follow-up samples, a multiplex master mix targeting exon 12 on the NPM1 gene is used to amplify DNA extracted from a patient sample. PCR products are sequenced to identify DNA sequences specific to previously identified mutations detected at diagnosis. Bioinformatics tools reliably detect specific sequences present at an allelic sensitivity level of 5 x 10-5.
Test Overview
Measurable residual disease (MRD) detection by Next-Generation Sequencing has demonstrated utility in predicting clinical outcomes and generating clinically actionable results. It allows early intervention, confirmation of disease status before transplant, and increased confidence in remission status.
Mutations in the nucleophosmin (NPM1) gene represent some of the most prevalent gene mutations in acute myeloid leukemia (AML).1 NPM1 mutations predominantly occur in AML with normal cytogenetics and are of prognostic value, especially within the context of FLT3 ITD mutations. Furthermore, because NPM1 displays a homogeneous mutation pattern, this gene represents an attractive target for MRD monitoring.2
MRD testing in patients with leukemia is helpful for the clinical management of the disease and can facilitate the development of new therapies.
Workflow
Key Benefits
- Baseline sample not required
- Faster turn-around with an in-house assay
- Reduce errors with streamlined workflow
- Software integrates into automated systems and LIMs
- Flexibility to multiplex up to 21 samples and multiple targets
THESE PRODUCTS ARE NOT AVAILABLE FOR PURCHASE BY THE GENERAL PUBLIC.
References:
- Falini, B et al. (2005) New England Journal of Medicine. 352:254–266.
- Krönke, J et al. (2011) Journal of Clinical Oncology. 29:2709-2716.
* Research Use Only. Not intended for diagnostic purposes.