Think Biocartis Idylla
WHEN TIME MATTERS. THINK BIOCARTIS IDYLLA*. YOUR BIOMARKER TEST RESULTS WITHIN 3 HOURS.
The Idylla™ solution is a revolutionary, fully automated, real-time PCR based molecular testing system designed to offer results in a minimum amount of time. Accessible on demand and without the need for batching. It makes molecular testing fast, convenient and suitable for any lab.
- Highly sensitive, fully automated biomarker testing.
- Less than 3 hours TAT.
- On solid and liquid biopsies.
- Minimal sample input requirements.
- 24/7 testing on single samples – No need for batching and loosing time.
- In-house testing suitable for any hospital lab setting – No need to send samples out.
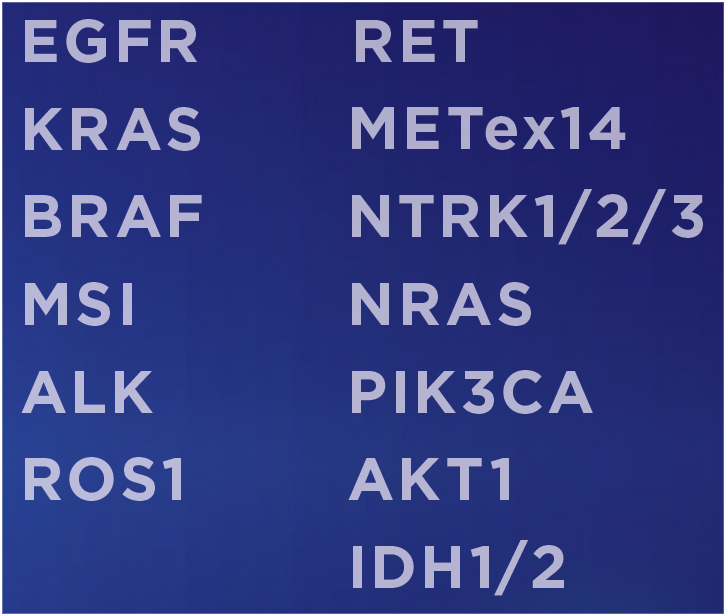
- Complementary to NGS.
Why IDYLLA™ FIRST?
- Idylla™ helps save lives. Patients die, rapidly deteriorate or receive sub-optimal treatment while waiting for their biomarker test results.2-4
- Idylla™ alleviates patient anxiety and allows them to focus on their well-being.5,6
- Idylla™ right first time. Idylla™ shows low failure rates combined with the ability to produce accurate results.1,7,8
- Idylla™ only requires small sample sizes. An NGS workflow needs nearly five times more tissue.2
References:
1 Behnke et al. FACILITATE A real-world multicenter prospective investigating the utility of fully automated real-time PCR assay versus local reference methods for detecting EGFR variants in NSCLC. Pathology & Oncology Research. Feb 2023
2 Finall et al. Integration of rapid PCR testing as an adjunct to NGS in diagnostic pathology services within the UK: evidence from a case series of nonsquamous, non-small cell lung cancer (NSCLC) patients with follow-up. J Clin Path. Jan 2022
3 Hardstock et al. Real-world treatment and survival of patients with advanced non-small cell lung Cancer: a German retrospective data analysis. BMC Cancer. Mar 2020
4 Sadik et al. Impact of Clinical Practice Gaps on the Implementation of Personalized Medicine in Advanced Non–Small-Cell Lung Cancer. JCO Precision Oncology. Oct 2022
5 Wheeldon et al. Use of the Biocartis Idylla™ Platform for the Detection of Epidermal Growth Factor Receptor, BRAF and KRAS Proto-Oncogene Mutations in Liquid-Based Cytology Specimens from Patients with Non-Small Cell Lung Carcinoma and Pancreatic Adenocarcinoma. J Mol Pathology. May 2022
6 Gregg et al. Molecular testing strategies in non-small cell lung cancer: Optimizing the diagnostic journey. Transl Lung Cancer Res. Jun 2019
7 Arcila et al. Ultra-Rapid EGFR Mutation Screening Followed by Comprehensive Next-Generation Sequencing: A Feasible, Informative Approach for Lung Carcinoma Cytology Specimens with a High Success Rate. JTO Clinical and Research Reports. Jul 2020
8 Chu et al. Clinical utility and performance of an ultra-rapid multiplex RNA-based assay for detection of ALK, ROS1, RET and NTRK1/2/3 rearrangements and MET exon 14 skipping alterations. J Mol Diag. Apr 2022
THESE PRODUCTS ARE NOT AVAILABLE FOR PURCHASE BY THE GENERAL PUBLIC.
* Check the regulatory status in your country.