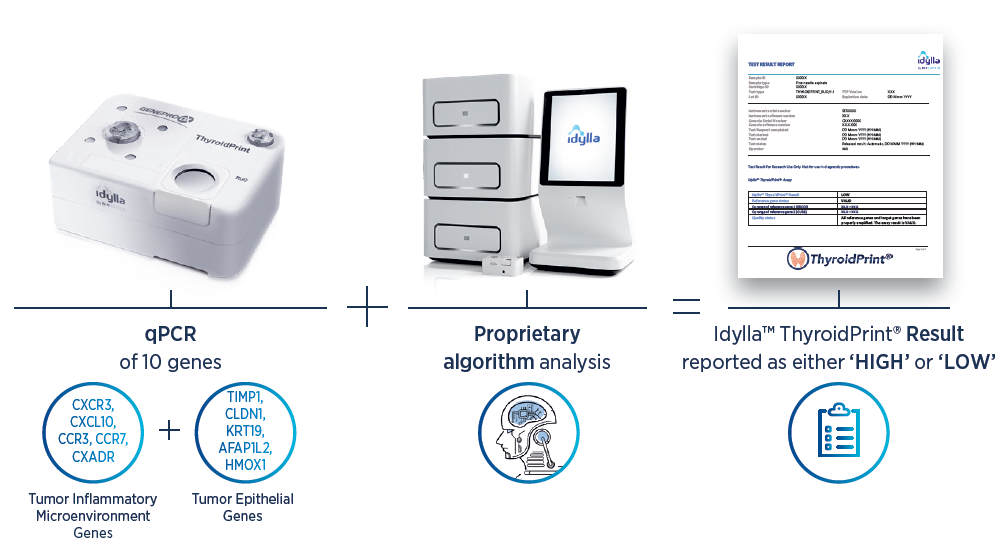
Biocartis – Idylla ThyroidPrint Assay
Idylla™ ThyroidPrint® Assay (RUO)* is a novel RT qPCR-based molecular signature that assesses a gene expression profile. Using a proprietary algorithm, ThyroidPrint provides assessment of indeterminate thyroid nodule cytology, classifying as low or high risk of malignancy.A ThyroidPrint low risk result empowers physicians to recommend watchful waiting instead of diagnostic surgery, therefore sparing patients from potential surgical risks and lifelong thyroid hormone supplementation.
Features of Idylla ThyroidPrint Assay
Strong scientific evidence for ThyroidPrint Assay:
- Test Design: Required Sensitivity 90% / Specificity 80%.1
- Gene Discovery & Prototype Development: 10-Gene Classifier: Sensitivity: 91% / Specificity 87%.2
- Multicenter Trials Clinical Validation: Proved Clinical Performance Sensitivity: 91% / Specificity 88%. Robust performance across ethnicities and genetic background.3
- Clinical Utility: Reduction of unnecessary surgery by 70%. Proven patient net-benefit.4
References:
1 Vargas-Salas S, Martínez JR, Urra S, et al. Genetic testing for indeterminate thyroid cytology: review and meta-analysis. Endocr Relat Cancer. 2018;25(3):R163-R177. doi:10.1530/ERC-17-0405
2 González HE, Martínez JR, Vargas-Salas S, et al. A 10-Gene Classifier for Indeterminate Thyroid Nodules: Development and Multicenter Accuracy Study. Thyroid. 2017;27(8):1058-1067. doi:10.1089/thy.2017.0067 www.mycancergenome.org
3 Zafereo M, McIver B, Vargas-Salas S, et al. A Thyroid Genetic Classifier Correctly Predicts Benign Nodules with Indeterminate Cytology: Two Independent, Multicenter, Prospective Validation Trials. Thyroid. 2020;30(5):704-712. doi:10.1089/thy.2019.0490
4 Domínguez et. al
* Research use only (RUO)
THESE PRODUCTS ARE NOT AVAILABLE FOR PURCHASE BY THE GENERAL PUBLIC.