27Sep
Thermo Fisher – 2023 new APS classification criteria
Last updated: 27th September, 2024
Thermo Fisher EliA testing for antiphospholipid syndrome (APS) in relation to the new 2023 APS classification criteria.
Why did EULAR/ACR update the APS classification criteria in 2023?1
- There were no evidence-based definitions incorporated in the revised 2006 criteria.
- New criteria ensure high-quality, risk-stratified epidemiologic studies and clinical trials.
- Maximising specificity can help to better understand disease pathophysiology and treatment effects.
Why are the new APS classification criteria relevant for you and your lab?
- The classification criteria are intended to classify patients for medical research purposes.2
- While the classification criteria were not developed for diagnosing APS, for facilitating and standardising. clinical research, they are often used as diagnostic criteria in the clinical setting.2
- Lab professionals often have only limited information about patient samples, not knowing if they are used for clinical studies or patient diagnosis.
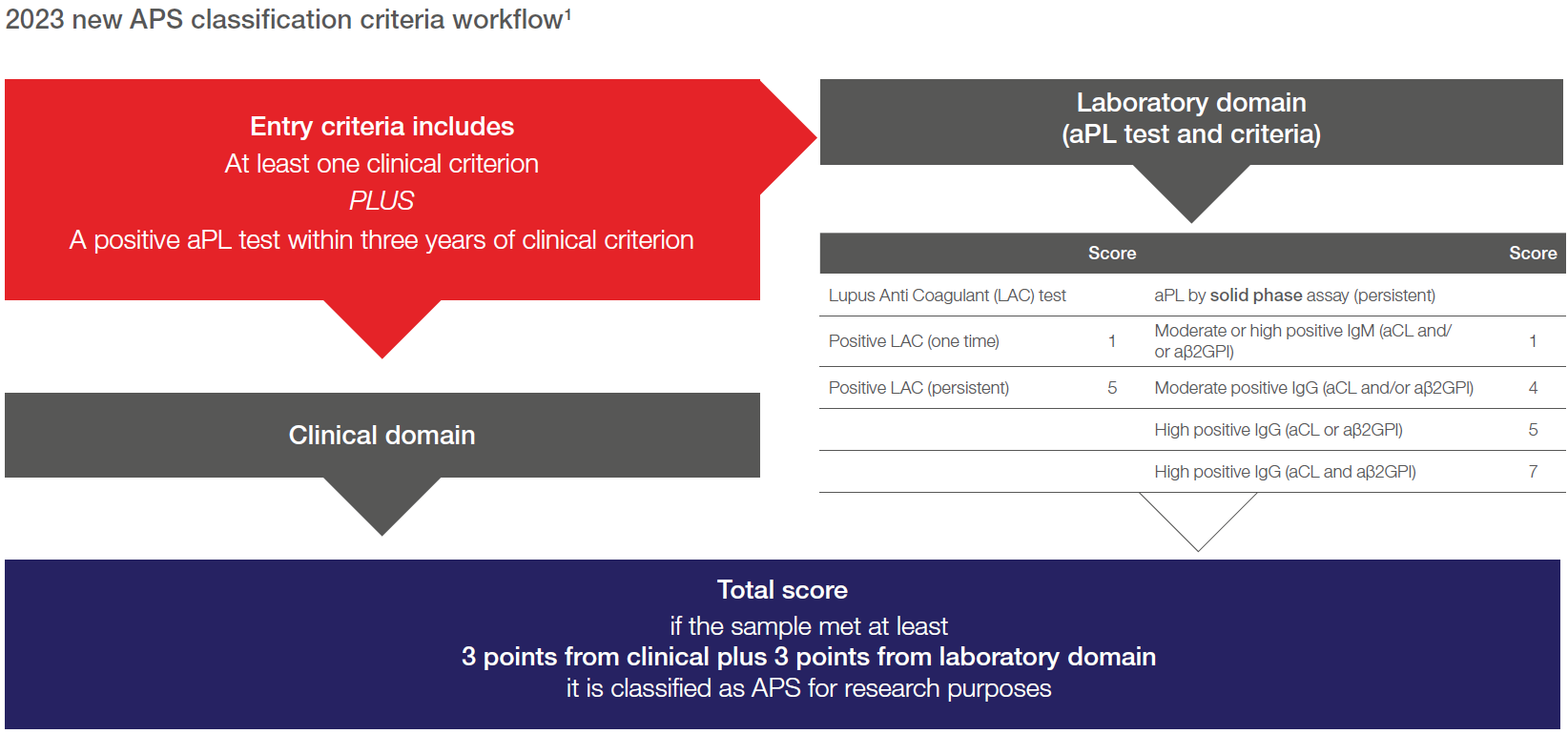
What are the key changes in the new APS classification criteria?¹
- Recommend delaying the use of the new automated platforms (chemiluminescence immunoassay, multiplex and flow cytometry) and instead using standardized enzyme-linked immunosorbent assay (ELISA) to determine antiphospholipid (aPL) antibodies.
- Evaluate in combination both IgG anti-cardiolipin (aCL) and IgG anti-ß2-glycoprotein (aß2GPI) positivity.
- Separately measure aCL/aß2GPI IgG and IgM isotypes.
- 2 levels of aCL/aβ2GPI positivity: moderate and high positive results based on ELISA technologies.
References:
1. Barbhaiya M. (2023) ACR/EULAR Antiphospholipid Syndrome Classification Criteria. Arthritis Rheumatol 75(10):1687-1702.
2. Vandevelde, A., & Devreese, K. M. J. (2022). Laboratory Diagnosis of Antiphospholipid Syndrome: Insights and Hindrances. Journal of clinical medicine, 11(8), 2164.
THESE PRODUCTS ARE NOT AVAILABLE FOR PURCHASE BY THE GENERAL PUBLIC.