Sebia – HYDRASHIFT 2/4 DARATUMUMAB Assay
THESE PRODUCTS ARE NOT AVAILABLE FOR PURCHASE BY THE GENERAL PUBLIC.
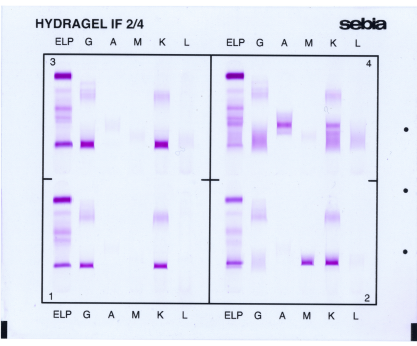
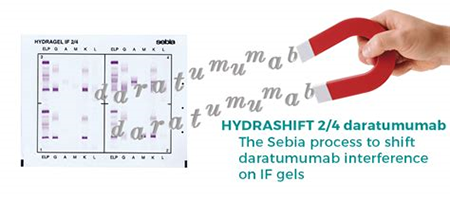
Sebia* HYDRASHIFT 2/4 DARATUMUMAB assay is intended to be used with HYDRAGEL IF, for the qualitative detection of monoclonal proteins in human serum by immunofixation electrophoresis.
Sebia is the world leader in diagnosis and monitoring of Multiple Myeloma patients by offering a full range of analytical solutions to enable clinicians to provide optimal medical care. Ensuring high-quality results adapted to all laboratories sizes. The Sebia solution offers serum and urine protein(s) electrophoresis (SPE/UPE) for screening and identification (immunofixation/Immunotyping), measurement of FLC and follow-up monoclonal antibody therapy with the HYDRASHIFT portfolio.
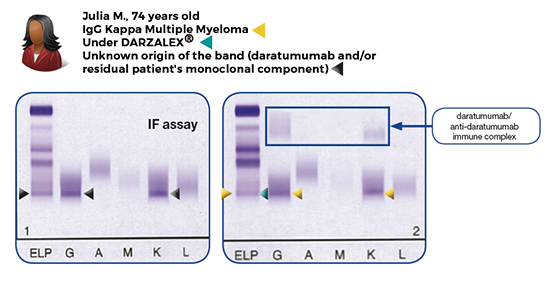
This in vitro diagnostic (IVD) reagent mitigates the daratumumab-mediated interference seen in Immunofixation results for patients with multiple myeloma treated with DARZALEX® (daratumumab), a fully human monoclonal antibody that binds to CD38.
The HYDRASHIFT 2/4 DARATUMUMAB Immunofixation assay is the result of a collaboration between Sebia (Paris, France) and Janssen Biotech, Inc. (Horsham, PA, USA), to provide the clinical community with better tools to monitor patients with multiple myeloma in line with the International Myeloma Working Group’s (IMWG) latest recommendations.
The HYDRASHIFT 2/4 DARATUMUMAB assay is performed on the Sebia HYDRASYS 2 agarose gel platform. The implementation of this assay will be easy and seamless in many of existing customer sites.
Brochures
Publications and websites
* Abacus dx is the exclusive distributor for Sebia in Australia only.