18Dec
SD Biosensor – Rapid Diagnostic Test: Standard Q Malaria P.f/Pan Ag Test
Last updated: 18th December, 2023
Products are for Professional/Laboratory use only
SD Biosensor Standard Q Rapid Diagnostic Malaria P.f/Pan Ag Test for IVD use.

STANDARD Q Malaria P.f/Pan Ag Test
- WHO Pre-Qualified & included on the ARTG for IVD use.
- Distinguishes the infection between P.falciparum and others.
- A cost-effective and fast malaria screening test.
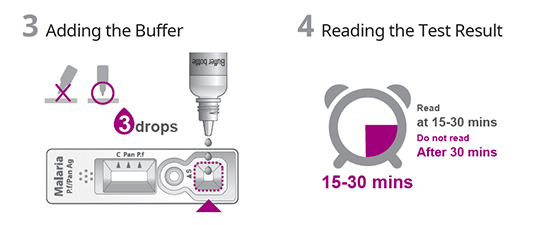
- Fast test result in 15 minutes.
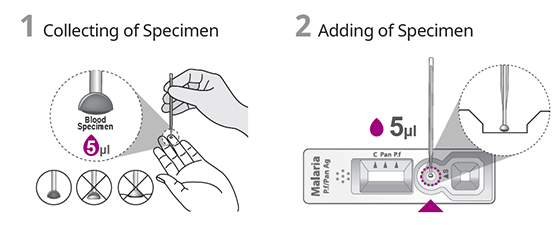
- Sampler (inverted cup) provided for easy specimen collection.
- Low volume of 5 μl whole blood required for testing.
- Room temperature storage of 2-40°C.
- Optimized for on-site diagnosis and practical for large scale testing.
PRODUCT INFORMATION
| STANDARD Q Malaria P.F/Pan Ag Test | |
| Target antigens | Histidine-Rich Protein II (HRP-II) of Plasmodium falciparum and plasmodium actate dehydrogenase (pLDH) of Plasmodium(P.f, P.v, P.m, P.o) species |
| Specimen type | Whole blood |
| Specimen Volume | 5 μl |
| Regulatory | WHO PQ, CE, TGA |
| Testing time | 15 ~ 30 minutes |
| Storage temperature | 2 ~ 40℃ (36 ~ 104℉) |
| Shelf life | 24 months |
PERFORMANCE CHARACTERISTICS
Clinical Evaluation
As part of WHO PQ clinical evaluation data, the evaluation took place in 4 sites (Cambodia, India, Malawia, Zambia) and the summary of the clinical performance is as below.
| Product | Specimen Type | Malaria Type | Sensitivity | Specificity |
| STANDARD Q Malaria P.f/Pan Ag Test | Venous WB | P.f | 99.58% (95% CI: 98.50-99.95%) |
100% (95% CI: 99.63-100%) |
| Pan | 100% (95% CI: 97.18-100%) |
100% (95% CI: 99.63-100%) |
||
| Capillary WB | P.f | 99.68% (95% CI: 98.23-99.99%) |
100% (95% CI: 98.54-100%) |
|
| Pan | 100% (95% CI: 88.78-100%) |
100% (95% CI: 98.54-100%) |
TEST PROCEDURE
ORDERING INFORMATION
| CAT No | Product | Type | Tests / Kit |
| 09MAL30D | STANDARD Q Malaria P.f/Pan Ag Test | Device | 25T / Kit |
| 10MALC10 | STANDARD Malaria Ag Control (RUO) | Control | 10T / Kit |