Merck SARS-CoV-2 ultrasensitive Single Molecule Counting SARS-CoV-2 RBD IgG Kit
Products are for professional/laboratory use only.
Immunoassay-based serology techniques play key roles in many SARS-CoV-2 research programs due to their ability to identify and characterize the immune response of individuals already infected by the virus through analysis of biomarkers such as immunoglobulins (Ig). However, the limited sensitivity and resolution offered by traditional assay platforms and their serology assays can leave unanswered crucial research questions.
Merck has harnessed the power of the Single Molecule Counting (SMC™) ultrasensitive immunoassay platform for the development of new products to address the scientific community’s needs including identifying low level SARS-CoV-2 mediated antibody response and resolving subtle differences in humoral immunity among individuals.
Traditional assay platform’s limitations in sensitivity and resolution, may not be truly optimal to assist researchers in accurately understanding the extent of SARS-CoV-2 infection in society. The new ultrasensitive SMC™ SARS-CoV-2 RBD IgG kit enables:
- Understanding the extent of immune heterogeneity among individuals and subpopulations.
- Thorough profile of immune response after natural or vaccine-induced exposure.
- Tracking persistence of long-term antibody COVID post-infection.
The new SMC™ SARS-CoV-2 RBD IgG kit can be used by researchers to accurately understand the extent of SARS-CoV-2 infection in society, thoroughly profile immune responses after vaccine challenge, and understand heterogeneous responses to infection among individuals and subpopulations. With unparalleled sensitivity and resolution the kit is designed to improve the value of data generated from crucial COVID-19 related vaccine development, epidemiology, and surveillance studies.
SMC™ SARS-CoV-2 RBD IgG Kit Characteristics include:
- Simple assay protocol (one hour capture, 30 minute detection steps)
- Detection of low level IgG host response against Receptor Binding Domain (RBD) of SARS-CoV-2 spike protein
- Ability to detect antibodies in 2 μL of human serum or plasma samples (within three days of positive PCR test)
- Rigorously developed to maximize sensitivity and specificity, while minimizing cross-reactivity
- Developed for the user-friendly SMCxPRO™ ultrasensitive immunoassay system
- Qualitative assay; for Research Use Only (RUO)
- Utility in vaccine development, epidemiology, and public health research
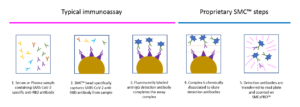
SMC™ SARS-CoV-2 RBD IgG Assay Mechanism
Assay mechanism includes proprietary elution and digital molecular counting steps that enable the SMC™ SARS-CoV-2 RBD IgG kit to detect antigen specific IgG in human serum and plasma at much lower levels compared to traditional immunoassays.
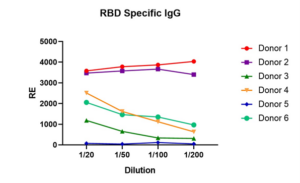
Example Data – Sample Titration
Instrument response (RE) when measuring titrations of plasma belonging to six donors on the SMCxPRO™ ultrasensitive immunoassay system using the SMC™ SARS-CoV-2 RBD IgG Kit. Sample dilution factor of 1:50 is recommended to maximize resolution of positive and negative results.
Example Data – Sensitivity, Specificity, and Cross Reactivity
Training Cohort Testing
|
Youden’s J Statistic |
||
|
Cutoff |
RE 400 |
RE 600 |
|
Sensitivity |
0.881 |
0.849 |
|
Specificity |
0.981 |
0.987 |
|
AUC |
0.981 |
0.981 |
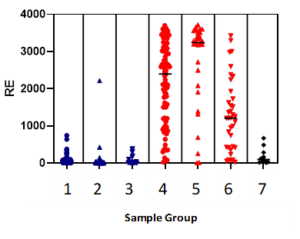
Aggregate data is shown for in-house testing of more than 300 donor plasma samples from seven training sample cohorts (1: pre-pandemic, 2 & 3: post-pandemic SARS-CoV-2 PCR negative, 4 to 6: post-pandemic SARS-CoV-2 PCR positive, 7: Influenza A/B positive samples for cross-reactivity testing), yielding high sensitivity and specificity when data is analyzed through Youden J’s Statistic using instrument response (RE) of 400 and 600 as example cut-points.
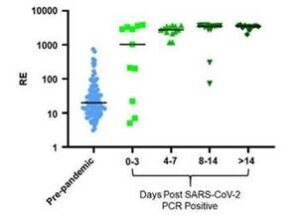
Example Data: Longitudinal Testing
SMC™ SARS-CoV-2 RBD-specific IgG kit performance in plasma from pre-pandemic and SARS-CoV-2 positive patients binned chronologically, demonstrating ability of this assay to identify immune response within 3 days of PCR positive result.
Analyte cross-reactivity
This assay was tested for recognition to SARS S RBD antigen, with 7% observed cross-reactivity detected.
Assay Conditions
Uses 100 µL per well of 1:50 diluted serum or plasma samples.
Performance Specifications
|
Precision in SMCxPRO™ Instrument Response |
||
|
Analyte |
Intra-Assay (% CV) |
Inter-Assay (% CV) |
|
SARS-CoV-2 RBD IgG |
14 |
21 |
SMC™ COVID-19 offerings include also range of verified high sensitivity protein biomarker kits verified high sensitivity protein biomarker kits
|
Description |
Order Number |
|
SMC™ SARS-CoV-2 RBD IgG Kit |
MP03019300 |
|
SMC™ SARS-CoV-2 S1 IgA Kit |
Coming Soon |
|
SMC™ SARS-CoV-2 RBD IgM Kit |
Coming Soon |
|
SMC™ Human cTnI High Sensitivity Immunoassay Kit |
MP03015400 |
|
SMC™ Human IL-6 High Sensitivity Immunoassay Kit |
MP03015500 |
|
SMC™ Human IL-4 High Sensitivity Immunoassay Kit |
MP03-016100 |
|
SMC™ Human TNFα High Sensitivity Immunoassay Kit |
MP03016300 |
|
SMC™ Human IFNα2 High Sensitivity Immunoassay Kit |
MP03018600 |
For Research Use Only. Not For Use In Diagnostic Procedures.