7 good reasons for choosing TissueSAFE plus over an industrial vacuum system
Products are for professional/laboratory use only.
1. CE IVD marking
The TissueSAFE plus is a certified CE-IVD (In-Vitro Diagnostic) medical device.
Its use is intended for biological specimens collection, transportation and preservation for up to 72 hours. The system with the related accessories and consumables are registered at the IVD devices registry of the Italian Ministry of Health.
Law strictly prohibits the utilization of a commercial or industrial device for a purpose that is not specifically indicated by its instructions for use, such as the operator manual. An improper use will invalidate the warranty and, in case of utilization of a device not marked CE-IVD for diagnostic purposes, it will expose the user to the legal risks arising from the incorrect handling/preservation of the biological material.
2. Scientific based
The TissueSAFE plus method allows the collection, transportation and preservetion of biological specimens in dedicated vacuum bags, kept at 4°C that have been scientifically validated by several independent medical organizations, such as the Italian Society of Pathology SIAPEC. There are over 25 scientific papers and posters showing the evidence of this method as applicable for all tissues on routine basis. The same kind of method performed with a different device will require equivalent scientific support and validation to be acceptable for routine use.
3. Hardware and software specifically designed
The TissueSAFE plus software and hardware are designed and calibrated to obtain the best performance, independent of the operators ability and skills. The vacuum level parameters created in the chamber are selected and controlled according to the type of tissue sealed in the vacuum bag. An improper pressure level or reduction in steps generated by a generic vacuum device would cause unrecoverable damage to biological specimens.
4. Safety features
The TissueSAFE plus is equipped with two filters mounted on the air exhaust outlet. The two filters are carbon and HEPA types to prevent the release into the air of pathogenic agents and particles coming from the air chamber extraction procedure. A generic vacuum device will expose the operators to high biological risks of contamination.
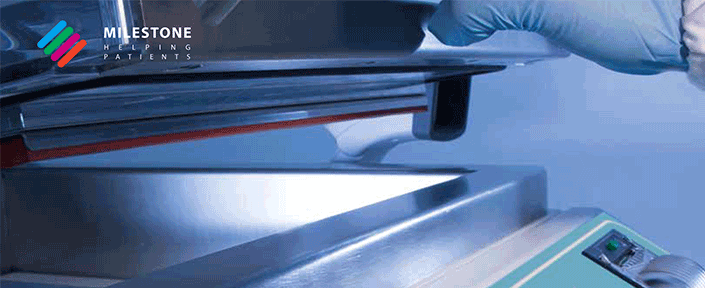
See the Milestone TissueSAFE in action:
f
5. Built-in printer
The TissueSAFE plus has a built-in printer to document the specimen ID, the starting of the vacuum process and the related parameters for full, complete traceability. With a generic vacuum device the above parameters are not included and are operator dependent.
6. Temperature/Time data logger
The TissueSAFE plus can be equipped with a built-in temperature monitoring kit, to control the temperature conditions of the specimens once sealed in the vacuum bags and during the transport, with printout on receipt into the laboratory.
7. Service and application support
The application and service support of Milestone and Abacus ALS are trained to grant you the full support: not just on the use and maintenance of the device itself, but also to support the implementation of the method for collecting, transporting and preserve biological specimens in vacuum bags. With a generic vacuum device the utilization and implementation on a routine basis will be under the entire responsibility of the final user.