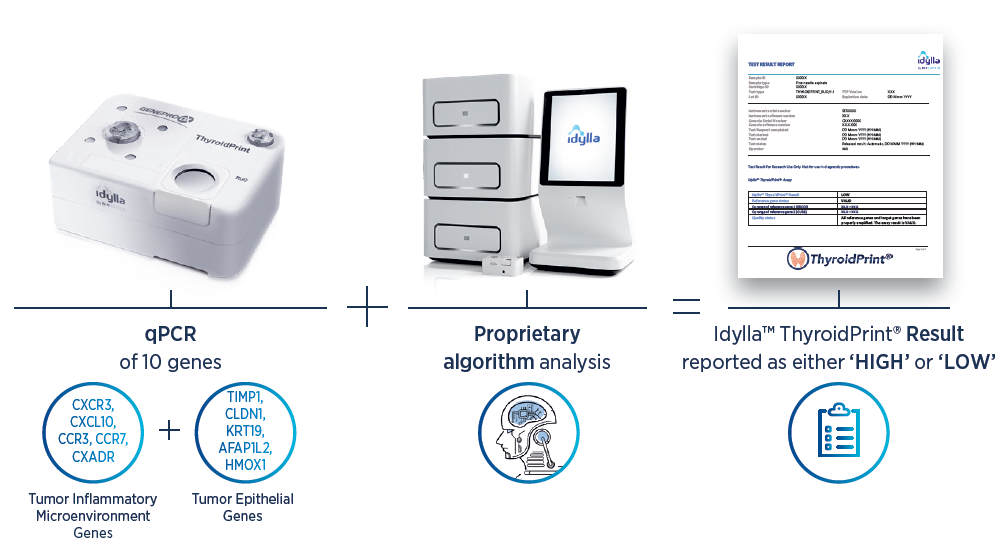
Biocartis – Idylla ThyroidPrint Assay
Idylla™ ThyroidPrint® Assay (RUO)* is a novel qPCR-based 10-gene classifier with its signature representing both the tumour microenvironment (CXCR3, CXCL10, CCR3, CCR7, and CXADR) and tumour epithelial cells (TIMP1, CLDN1, KTR19, AFAPL2, and HMOX1). The signature’s qPCR data is analysed by a neural network algorithm that predicts the probability of benign indeterminate thyroid nodules.
A benign test result allows physicians to recommend watchful waiting as an alternative to diagnostic surgery. This prevents exposing patients to surgical risks and permanent thyroid hormone supplementation. Moreover, it significantly reduces health costs associated with unnecessary surgery.
Features of Idylla ThyroidPrint Assay
Strong scientific evidence for ThyroidPrint Assay:
- Test Design: Required Sensitivity 90% / Specificity 80%.1
- Gene Discovery & Prototype Development: 10-Gene Classifier: Sensitivity: 91% / Specificity 87%.2
- Multicenter Trials Clinical Validation: Proved Clinical Performance Sensitivity: 91% / Specificity 88%. Robust performance across ethnicities and genetic background.3
- Clinical Utility: Reduction of unnecessary surgery by 70%. Proven patient net-benefit.4
References:
1 Vargas-Salas S, Martínez JR, Urra S, et al. Genetic testing for indeterminate thyroid cytology: review and meta-analysis. Endocr Relat Cancer. 2018;25(3):R163-R177. doi:10.1530/ERC-17-0405
2 González HE, Martínez JR, Vargas-Salas S, et al. A 10-Gene Classifier for Indeterminate Thyroid Nodules: Development and Multicenter Accuracy Study. Thyroid. 2017;27(8):1058-1067. doi:10.1089/thy.2017.0067 www.mycancergenome.org
3 Zafereo M, McIver B, Vargas-Salas S, et al. A Thyroid Genetic Classifier Correctly Predicts Benign Nodules with Indeterminate Cytology: Two Independent, Multicenter, Prospective Validation Trials. Thyroid. 2020;30(5):704-712. doi:10.1089/thy.2019.0490
4 Domínguez et. al
* Research use only (RUO)
THESE PRODUCTS ARE NOT AVAILABLE FOR PURCHASE BY THE GENERAL PUBLIC.