Devyser NIPT RHD
Benefit from proven, highly sensitive technology to conduct accurate non-invasive screening for fetal RHD that helps clinicians avoid untargeted and unnecessary treatment.
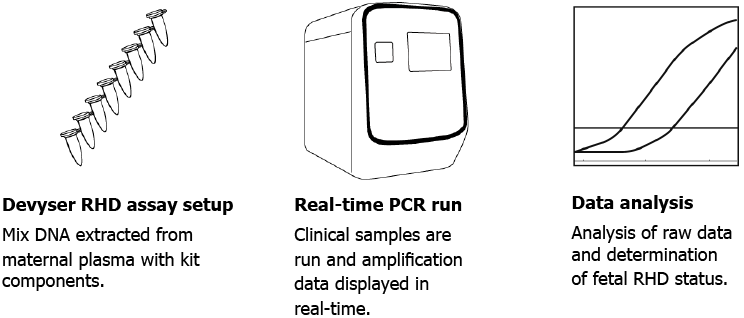
Devyser NIPT RHD is a test kit used by clinical labs to determine fetal RHD status from maternal plasma as early as gestation week 10. Devyser’s unique single exon design significantly simplifies your laboratory workflow and analysis. It also enables you to increase throughput turnaround times. Diagnostic sensitivity and specificity are both ≥99.86%, as measured by correlation to the newborn’s rhesus serology.
Simplified testing with high sensitivity
A single exon analysis (exon 4) allows non-invasive detection of fetal RHD status from gestation week 10. The assay design simplifies laboratory work, automation and analysis.
Quick start and cost control
All required PCR reagents are included in Devyser’s kit. You can get started immediately and have complete control of your costs.
“The single-exon design results in high sensitivity and simple response algorithms.”
Dr. Agneta Wikman, M.D. Associate Professor Karolinska University Hospital, Stockholm
Key features and benefits of Devyser RHD
- Testing from gestation week 10
- Sensitive and specific RHD determination
- Single exon design
- Easy to automate
- Use of maternal plasma
- Stratify your patients very early
- Treat only the RHD-negative mothers at risk
- Run fewer reactions per sample
- Reduce risk with streamlined workflow
- Non-invasive test
* Included on the Australian Register for Therapeutic Goods (ARTG)
THESE PRODUCTS ARE NOT AVAILABLE FOR PURCHASE BY THE GENERAL PUBLIC.